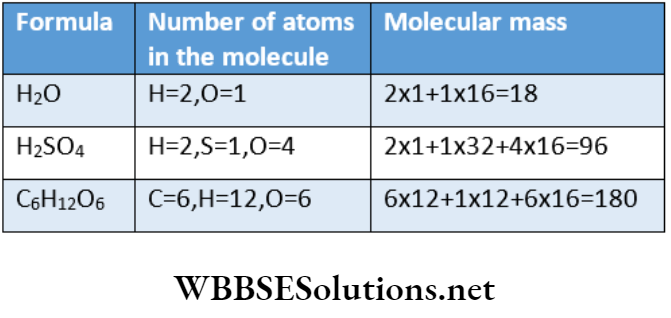
5.85g of NaCl is dissolved in 1L of pure water. The number of ions
Par un écrivain mystérieux
Last updated 08 juillet 2024

5.85g of NaCl is dissolved in 1L of pure water. The number of ions in 1ml of this solution is
Vasantha, Author at WBBSE Solutions

A solution containing 0.5g of KCl dissolved in 100g of water and freezes { 0.24 }^{ o }C. Calculate the degree of dissociation of the salt. ({K}_{f} water ={ 1.86 }^{ o }
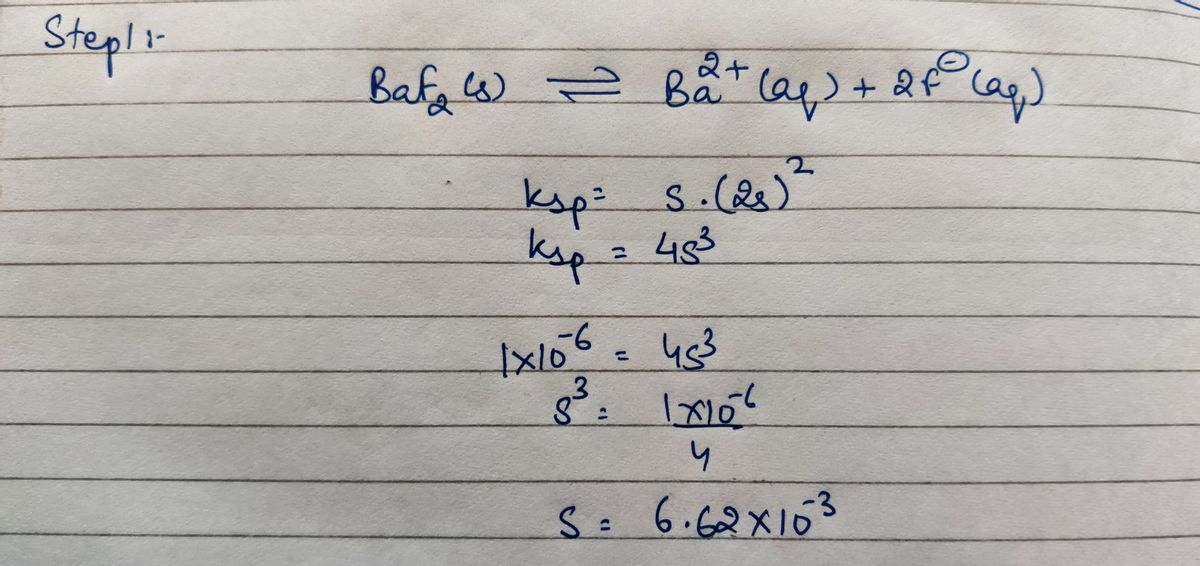
Answered: How many grams of BaF2 (molar mass =…
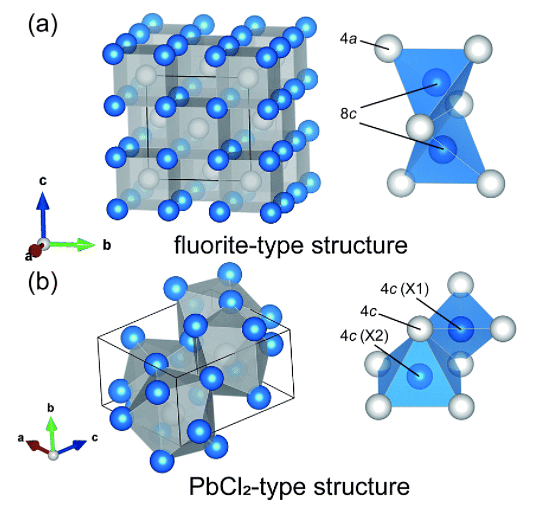
Lead II Chloride Formula: Properties, Structure, Examples
5.85g of NaCl is dissolved in 200ml of water. What will be the molarity of the solution? - Quora
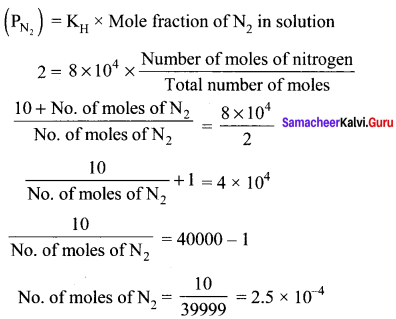
Samacheer Kalvi 11th Chemistry Solutions Chapter 9 Solutions – Samacheer Kalvi
What will be the mass of sodium chloride formed when 5.3 g of sodium carbonate is dissolved in 250 ml of a half molar HCl solution? - Quora
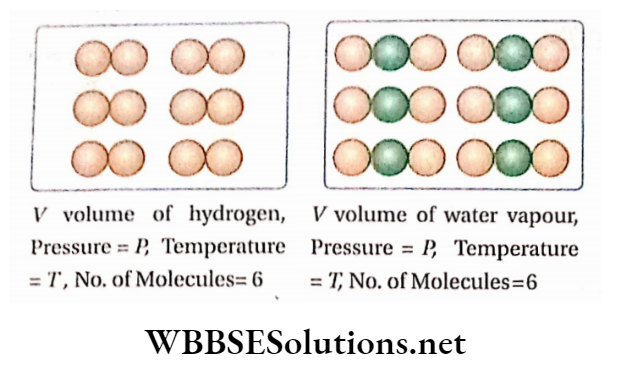
Vasantha, Author at WBBSE Solutions

FINAL fiit-jee SOME BASIC CONCEPT..docx
Recommandé pour vous
 Versylène Nacl 1L Lavage de Nez et Irrigations Grande bouteille Sérum Phy14 Jul 2023
Versylène Nacl 1L Lavage de Nez et Irrigations Grande bouteille Sérum Phy14 Jul 2023 Saline (medicine) - Wikipedia14 Jul 2023
Saline (medicine) - Wikipedia14 Jul 2023 Sodium Chlorure 0,9% Stérile Apyrogène Otec 1 L (Carton De 1014 Jul 2023
Sodium Chlorure 0,9% Stérile Apyrogène Otec 1 L (Carton De 1014 Jul 2023 B Braun IV Infusion Sodium Chloride (NaCl) 1L14 Jul 2023
B Braun IV Infusion Sodium Chloride (NaCl) 1L14 Jul 2023 Pharmamed - NACL14 Jul 2023
Pharmamed - NACL14 Jul 2023 Sodium Chloride 0.9% for Irrigation 1L - Dental Medical Ireland14 Jul 2023
Sodium Chloride 0.9% for Irrigation 1L - Dental Medical Ireland14 Jul 2023 Baxter Saline - Sodium Chloride (NaCl) 0.9%14 Jul 2023
Baxter Saline - Sodium Chloride (NaCl) 0.9%14 Jul 2023 Saline Irrigation Water Baxter Nacl 0.9% 1 Litre 1000ml Bottle14 Jul 2023
Saline Irrigation Water Baxter Nacl 0.9% 1 Litre 1000ml Bottle14 Jul 2023 RICCA Nacl Cond Std, 5 S/Cm Size (1 L)14 Jul 2023
RICCA Nacl Cond Std, 5 S/Cm Size (1 L)14 Jul 2023 Silver Nitrate, (1mL = 10mg NaCl), Certified, 0.171N 0.002N (0.17114 Jul 2023
Silver Nitrate, (1mL = 10mg NaCl), Certified, 0.171N 0.002N (0.17114 Jul 2023
Tu pourrais aussi aimer
 Pinata licorne My Little Day - Anniversaire licorne14 Jul 2023
Pinata licorne My Little Day - Anniversaire licorne14 Jul 2023 SEADESKY 12 Rouleaux Bandage Autoadhésif Cohésifs 2.5cm Bande Cohés14 Jul 2023
SEADESKY 12 Rouleaux Bandage Autoadhésif Cohésifs 2.5cm Bande Cohés14 Jul 2023 Cadeau mamie - Mug personnalisé supers pouvoirs - La boite à Mug14 Jul 2023
Cadeau mamie - Mug personnalisé supers pouvoirs - La boite à Mug14 Jul 2023 doublure d'étang de remblai de l'étang Liner/HDPE Geomembrane de14 Jul 2023
doublure d'étang de remblai de l'étang Liner/HDPE Geomembrane de14 Jul 2023 Ceinture d'entraînement de sport – Dovalux14 Jul 2023
Ceinture d'entraînement de sport – Dovalux14 Jul 2023- J. Lynn Cosmetics14 Jul 2023
 Cyclone Boys Metallic 4x4 M – SpeedyCubes14 Jul 2023
Cyclone Boys Metallic 4x4 M – SpeedyCubes14 Jul 2023![Adaptateur Prise Anglaise UK Angleterre Française Adaptateur de Voyage avec 1 USB-A et 1 USB-C 2.4A,Europe Francaise FR 2 Broc[126]](https://www.cdiscount.com/pdt2/9/5/9/1/700x700/auc3094865679959/rw/adaptateur-prise-anglaise-uk-angleterre-francaise.jpg) Adaptateur Prise Anglaise UK Angleterre Française Adaptateur de Voyage avec 1 USB-A et 1 USB-C 2.4A,Europe Francaise FR 2 Broc[126]14 Jul 2023
Adaptateur Prise Anglaise UK Angleterre Française Adaptateur de Voyage avec 1 USB-A et 1 USB-C 2.4A,Europe Francaise FR 2 Broc[126]14 Jul 2023 250 Feuilles A4 250 Feuilles blanches 250 Feuilles Blanches pour pochettes A4 horizontales - ImpressionMenu14 Jul 2023
250 Feuilles A4 250 Feuilles blanches 250 Feuilles Blanches pour pochettes A4 horizontales - ImpressionMenu14 Jul 2023 FIDGET TOY BOX PLUS💛 (Monthly) – Box of Sensory Toys14 Jul 2023
FIDGET TOY BOX PLUS💛 (Monthly) – Box of Sensory Toys14 Jul 2023
