Report from the Advisory Committee on Immunization Practices (ACIP): Decision Not to Recommend Routine Vaccination of All Children Aged 2--10 Years with Quadrivalent Meningococcal Conjugate Vaccine (MCV4)
Par un écrivain mystérieux
Last updated 05 juillet 2024
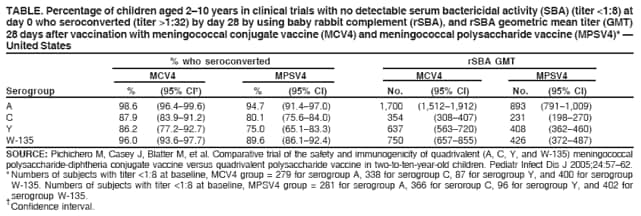

Meningococcal ACWY

ACIP Recommends New Penbraya Vaccine Be Added to Vaccine Schedule

PDF] Meningococcal Vaccination: Recommendations of the Advisory Committee on Immunization Practices, United States, 2020

Rationale for the Development of a Pentavalent Meningococcal Vaccine: A US-Focused Review
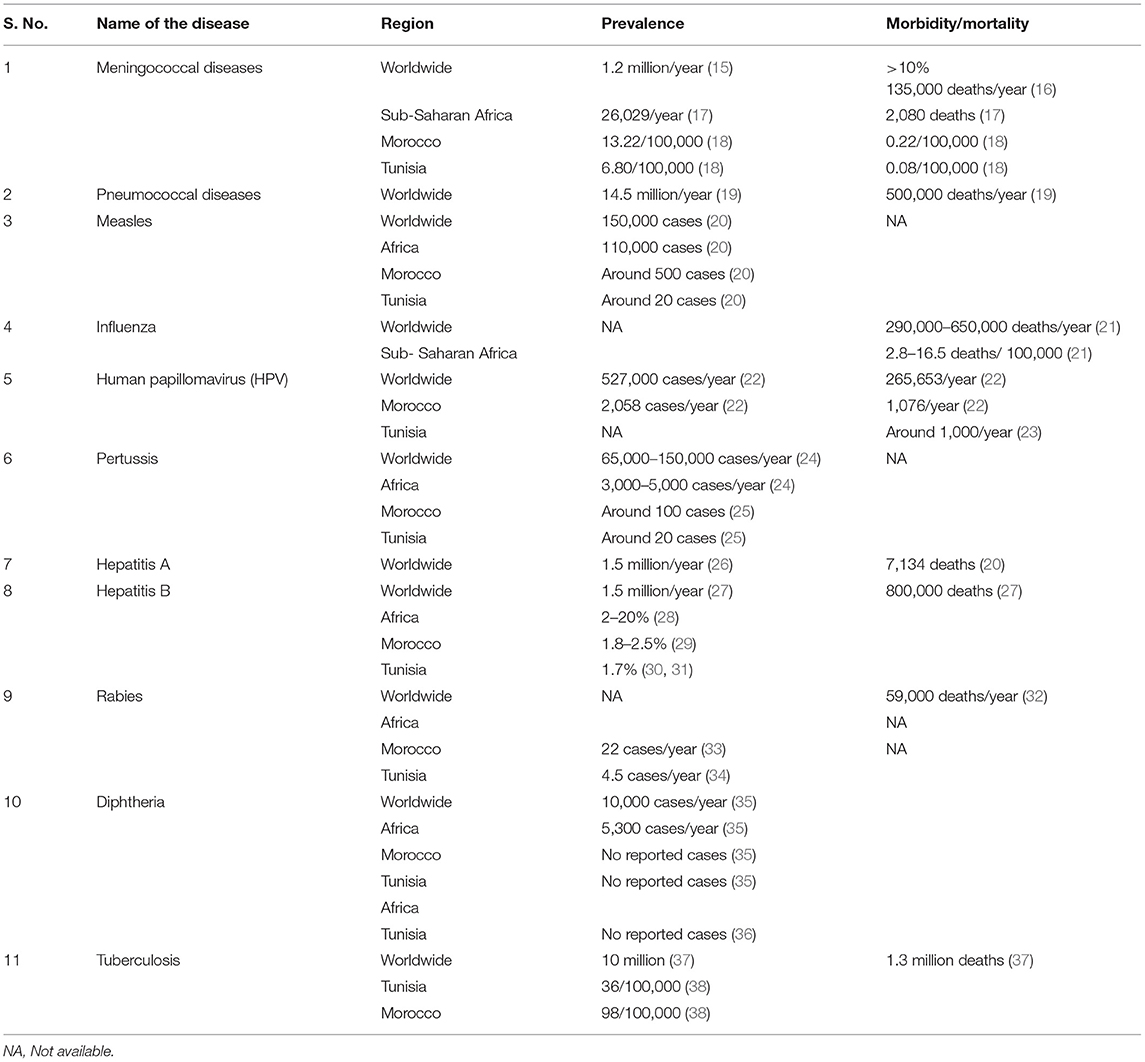
Frontiers Trends in Adult and Elderly Vaccination: Focus on Vaccination Practices in Tunisia and Morocco

Pediatric Vaccinations: Do You Know the Recommended Schedules?
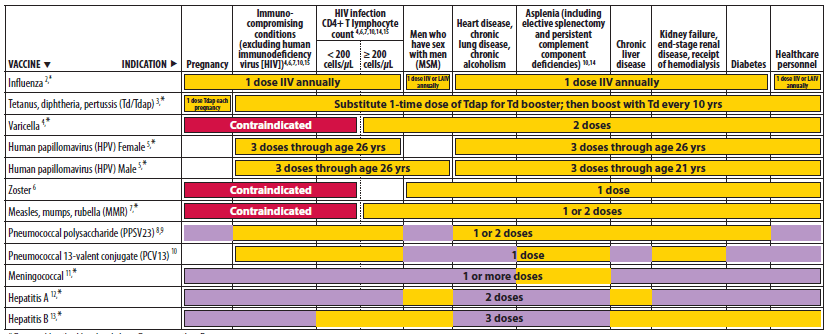
Advisory Committee on Immunization Practices (ACIP) Recommended Immunization Schedule for Adults Aged 19 Years and Older — United States, 2013
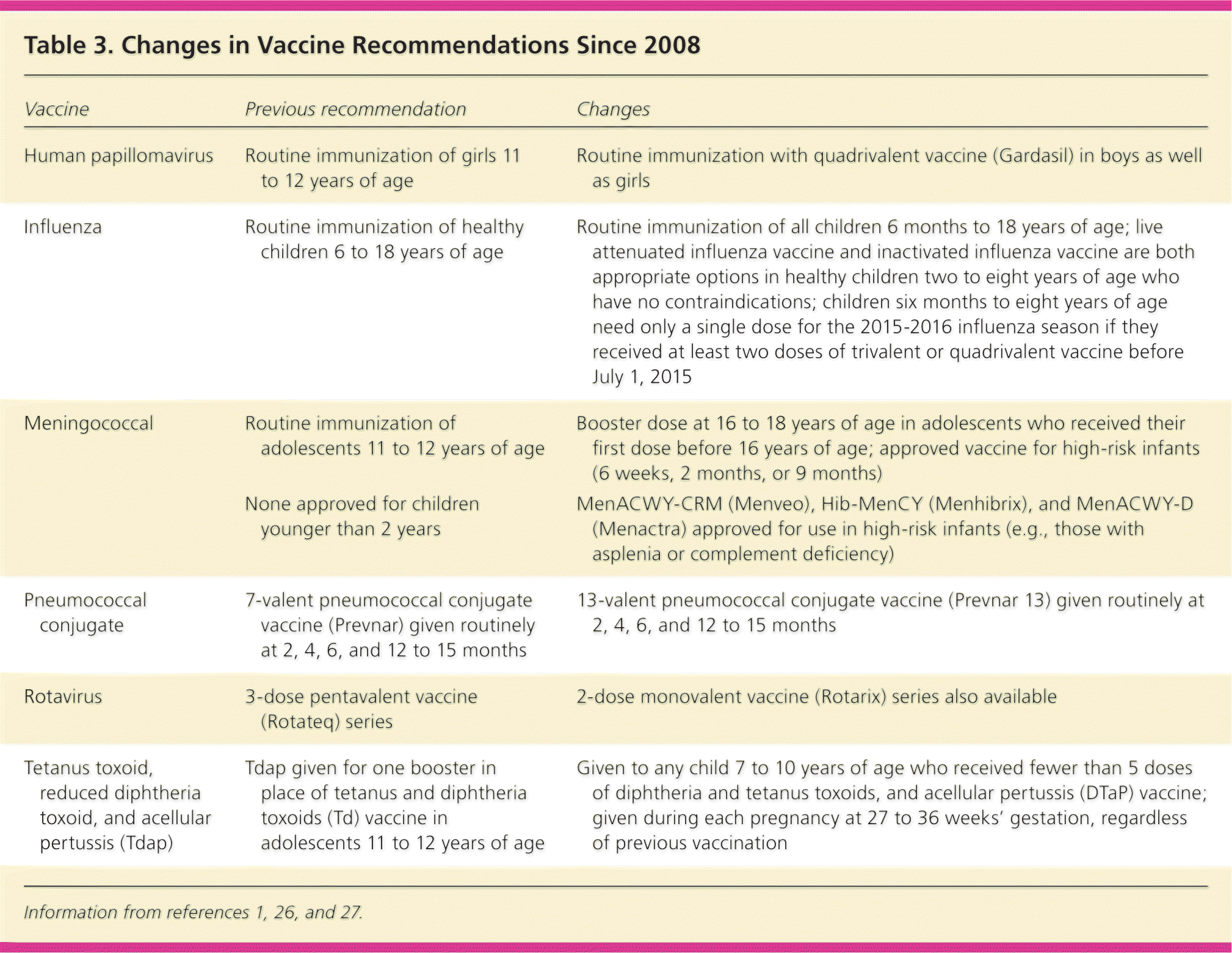
Update on Routine Childhood and Adolescent Immunizations
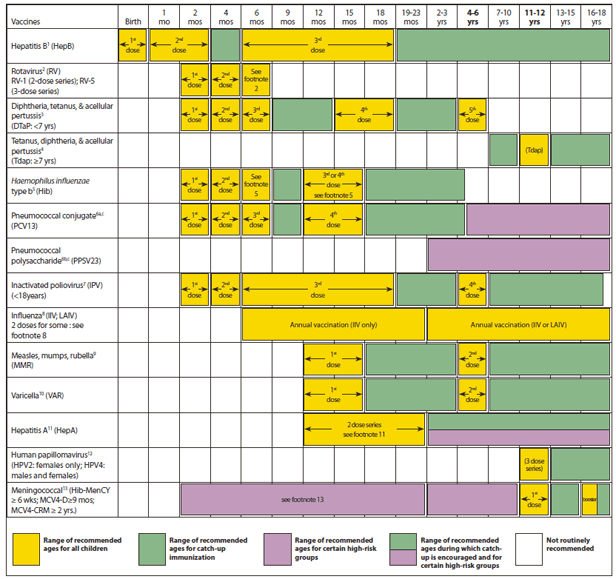
Advisory Committee on Immunization Practices (ACIP) Recommended Immunization Schedule for Persons Aged 0 Through 18 Years — United States, 2013

Full article: Safety and immunogenicity of co-administered meningococcal serogroup B (4CMenB) vaccine: A literature review
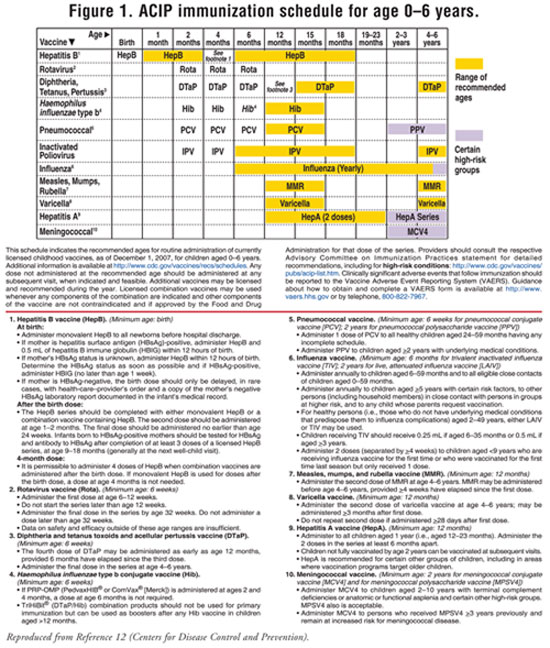
Pediatric and Adolescent Vaccines Update

Biomedicines, Free Full-Text
Recommandé pour vous
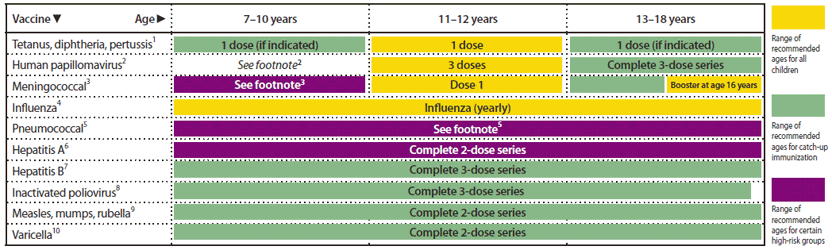 Recommended Immunization Schedules for Persons Aged 0 Through 18 Years — United States, 201214 Jul 2023
Recommended Immunization Schedules for Persons Aged 0 Through 18 Years — United States, 201214 Jul 2023 Get protected14 Jul 2023
Get protected14 Jul 2023 Meningitis :: Understanding Animal Research14 Jul 2023
Meningitis :: Understanding Animal Research14 Jul 2023- Dr Salman Bajwa - By the grace of God, separate portal for14 Jul 2023
 Rx Item-Menactra Meningitis Vaccine 4Mcg 0.5Ml Vial 5X1 By Sanofi Pasteur14 Jul 2023
Rx Item-Menactra Meningitis Vaccine 4Mcg 0.5Ml Vial 5X1 By Sanofi Pasteur14 Jul 2023 Table 4 from Adolescent immunizations.14 Jul 2023
Table 4 from Adolescent immunizations.14 Jul 2023 Doepfer - MCV4 MIDI-to-CV Interface – Noisebug14 Jul 2023
Doepfer - MCV4 MIDI-to-CV Interface – Noisebug14 Jul 2023 Full article: Comparing the meningococcal serogroup C immune response elicited by a tetanus toxoid conjugate quadrivalent meningococcal vaccine (MenACYW-TT) versus a quadrivalent or monovalent C tetanus toxoid conjugate meningococcal vaccine in healthy14 Jul 2023
Full article: Comparing the meningococcal serogroup C immune response elicited by a tetanus toxoid conjugate quadrivalent meningococcal vaccine (MenACYW-TT) versus a quadrivalent or monovalent C tetanus toxoid conjugate meningococcal vaccine in healthy14 Jul 2023 New law requires Ga. 11th-graders to get meningitis shot14 Jul 2023
New law requires Ga. 11th-graders to get meningitis shot14 Jul 2023/https://static.texastribune.org/media/images/2013/08/08/Vaccine-Lead.jpg) Meningitis Vaccine Rules at Colleges to Be Scaled Back14 Jul 2023
Meningitis Vaccine Rules at Colleges to Be Scaled Back14 Jul 2023
Tu pourrais aussi aimer
 Totem Pole14 Jul 2023
Totem Pole14 Jul 2023 Gilet Sécurité matelassé noir14 Jul 2023
Gilet Sécurité matelassé noir14 Jul 2023 Table à langer avec baignoire coulissante Baby Pool + Matelas à lang – roba14 Jul 2023
Table à langer avec baignoire coulissante Baby Pool + Matelas à lang – roba14 Jul 2023 HIKENTURE Coussin Gonflable de Camping/Voyage avec taie d'oreiller Amovible, Coussin Ergonomique, Coussin de Nuque Confortable pour Voyage/extérieur, Coussin Gonflable pour Voyage et Voyage-Bleu : : Sports et Loisirs14 Jul 2023
HIKENTURE Coussin Gonflable de Camping/Voyage avec taie d'oreiller Amovible, Coussin Ergonomique, Coussin de Nuque Confortable pour Voyage/extérieur, Coussin Gonflable pour Voyage et Voyage-Bleu : : Sports et Loisirs14 Jul 2023 Sangle de maintien14 Jul 2023
Sangle de maintien14 Jul 2023 AUTOPOWERZ LED Headlight Bulb only with H4 Fitting 40 WATT Hi/Low Beam, Only for Bikes : : Car & Motorbike14 Jul 2023
AUTOPOWERZ LED Headlight Bulb only with H4 Fitting 40 WATT Hi/Low Beam, Only for Bikes : : Car & Motorbike14 Jul 2023 Visière casque Chase jaune Roeg14 Jul 2023
Visière casque Chase jaune Roeg14 Jul 2023 iPhone 4/4S - iPhone - SHOP14 Jul 2023
iPhone 4/4S - iPhone - SHOP14 Jul 2023 Crédence Adhésive - Carreaux de ciment - Perrine - Mix Bleu - 40x200cm - Vinyle Adhésif14 Jul 2023
Crédence Adhésive - Carreaux de ciment - Perrine - Mix Bleu - 40x200cm - Vinyle Adhésif14 Jul 2023 Yum Asia Panda Mini Rice Cooker14 Jul 2023
Yum Asia Panda Mini Rice Cooker14 Jul 2023
