AAFP Backs New EUAs on COVID-19 Boosters for Children
Par un écrivain mystérieux
Last updated 06 juillet 2024

The AAFP reviewed evidence on the use of bivalent COVID-19 booster vaccines in children and adolescents, and approved action by the FDA and CDC on amended emergency use authorizations.

AAFP Approves Federal Actions on COVID-19 Boosters
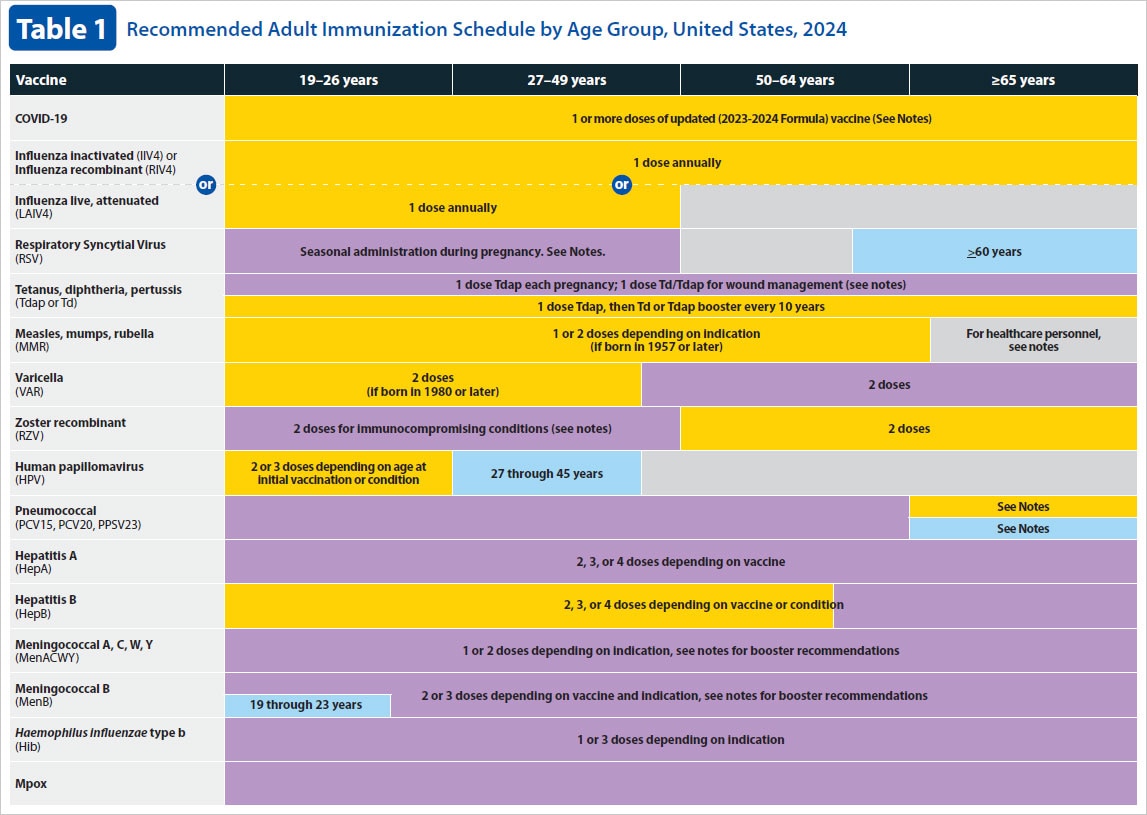
Adult Immunization Schedule – Healthcare Providers

F.D.A. Authorizes Updated Covid Shots for Children as Young as 6 Months - The New York Times

AAFP Approves Updated COVID-19 Vaccine Plan From FDA, CDC

AAFP Supports Expanded EUAs on Bivalent COVID-19 Boosters

FDA Authorizes COVID Vaccine Boosters For Kids 12 To 15 Years Old - CBS Miami

Vaccines & Diseases - Vaccinate Your Family

Fourth dose boosters and pediatric vaccines for COVID-19

AAFP Backs New EUAs on COVID-19 Boosters for Children

FDA Authorizes Updated Covid Booster Shots For Kids 5 to 11 - The New York Times
Recommandé pour vous
 Génération Yamaha BW's et MBK Booster14 Jul 2023
Génération Yamaha BW's et MBK Booster14 Jul 2023![MBK [Booster] Après 2004](https://www.bcd-design.com/modules/totcustomfields/uploads/image/e4fb60156dbdff25936b1f1895a60c9d.jpg) MBK [Booster] Après 200414 Jul 2023
MBK [Booster] Après 200414 Jul 2023 COVID-19 Boosters: Why You Should Schedule One Now14 Jul 2023
COVID-19 Boosters: Why You Should Schedule One Now14 Jul 2023 Testosterone Booster, Pills for Power & Strength14 Jul 2023
Testosterone Booster, Pills for Power & Strength14 Jul 2023 Resource Booster, WARFRAME Wiki14 Jul 2023
Resource Booster, WARFRAME Wiki14 Jul 2023 Latest generation 12/24 V Lithium Booster Starter - Gen-Art14 Jul 2023
Latest generation 12/24 V Lithium Booster Starter - Gen-Art14 Jul 2023 Marine Octane Booster – BOOSTane14 Jul 2023
Marine Octane Booster – BOOSTane14 Jul 2023 Ooze Booster Extract Vaporizer14 Jul 2023
Ooze Booster Extract Vaporizer14 Jul 2023 Booster Raises $125M+ in Funding to Expand Mobile Fuel Delivery, Accelerating Decarbonization of the Mobility and Transportation Sector14 Jul 2023
Booster Raises $125M+ in Funding to Expand Mobile Fuel Delivery, Accelerating Decarbonization of the Mobility and Transportation Sector14 Jul 2023 Building A Booster Club14 Jul 2023
Building A Booster Club14 Jul 2023
Tu pourrais aussi aimer
 Mille Baisers pour un garçon - version collector avec14 Jul 2023
Mille Baisers pour un garçon - version collector avec14 Jul 2023 SALON MAROCAIN PAS LOIN DE MARSEILLE TEL: 06 09 46 93 5214 Jul 2023
SALON MAROCAIN PAS LOIN DE MARSEILLE TEL: 06 09 46 93 5214 Jul 2023![Jouet Bain Bébé 1 2 3 4+ Ans, Jeu Bain Bebe Avec Ventouses, Jeux Bain À Glissière Jeux D'Eau Rotatifs Watermill, Jouet Bain[P177] - Cdiscount Puériculture & Eveil bébé](https://www.cdiscount.com/pdt2/3/7/9/3/700x700/tra1689355792379/rw/jouet-bain-bebe-1-2-3-4-ans-jeu-bain-bebe-avec-v.jpg) Jouet Bain Bébé 1 2 3 4+ Ans, Jeu Bain Bebe Avec Ventouses, Jeux Bain À Glissière Jeux D'Eau Rotatifs Watermill, Jouet Bain[P177] - Cdiscount Puériculture & Eveil bébé14 Jul 2023
Jouet Bain Bébé 1 2 3 4+ Ans, Jeu Bain Bebe Avec Ventouses, Jeux Bain À Glissière Jeux D'Eau Rotatifs Watermill, Jouet Bain[P177] - Cdiscount Puériculture & Eveil bébé14 Jul 2023 MINIDOU ASSOUPLISSANT14 Jul 2023
MINIDOU ASSOUPLISSANT14 Jul 2023 Calculatrice de bureau CASIO modèle FR-2650 RC14 Jul 2023
Calculatrice de bureau CASIO modèle FR-2650 RC14 Jul 2023 Colorfabb lance l'impression 3D à base de bronze et de bambou - 3Dnatives14 Jul 2023
Colorfabb lance l'impression 3D à base de bronze et de bambou - 3Dnatives14 Jul 2023- Filetage Et Taraudage Cours PDF, PDF, Vis de fixation14 Jul 2023
![15 Pcs Aiguilles à Coudre Aiguilles Emoussées Aiguille à Repriser à Grand Œil avec Dé à Coudre et Enfile-Aiguille pour Laine [144] - Cdiscount Beaux-Arts et Loisirs créatifs](https://www.cdiscount.com/pdt2/0/9/7/1/700x700/auc3455671197097/rw/15-pcs-aiguilles-a-coudre-aiguilles-emoussees-aigu.jpg) 15 Pcs Aiguilles à Coudre Aiguilles Emoussées Aiguille à Repriser à Grand Œil avec Dé à Coudre et Enfile-Aiguille pour Laine [144] - Cdiscount Beaux-Arts et Loisirs créatifs14 Jul 2023
15 Pcs Aiguilles à Coudre Aiguilles Emoussées Aiguille à Repriser à Grand Œil avec Dé à Coudre et Enfile-Aiguille pour Laine [144] - Cdiscount Beaux-Arts et Loisirs créatifs14 Jul 2023 Avis / test - MIXMEST@ 180 x 60 x 5 cm - Tapis de Gymnastique Pliable - pour Gym, Yoga, Fitness à la Maison - BLEU CLAIR!!! - AUCUNE - Prix14 Jul 2023
Avis / test - MIXMEST@ 180 x 60 x 5 cm - Tapis de Gymnastique Pliable - pour Gym, Yoga, Fitness à la Maison - BLEU CLAIR!!! - AUCUNE - Prix14 Jul 2023 Mesureur de cuisine de 1,0 L │ Leifheit14 Jul 2023
Mesureur de cuisine de 1,0 L │ Leifheit14 Jul 2023
