Does The Difference In Structure Make Graphite Soft But Diamond Hard?
Par un écrivain mystérieux
Last updated 24 juillet 2024


Diamond vs. Graphite: What is the Difference?

Consider the following statements : 1. Diamond is hard and graphite
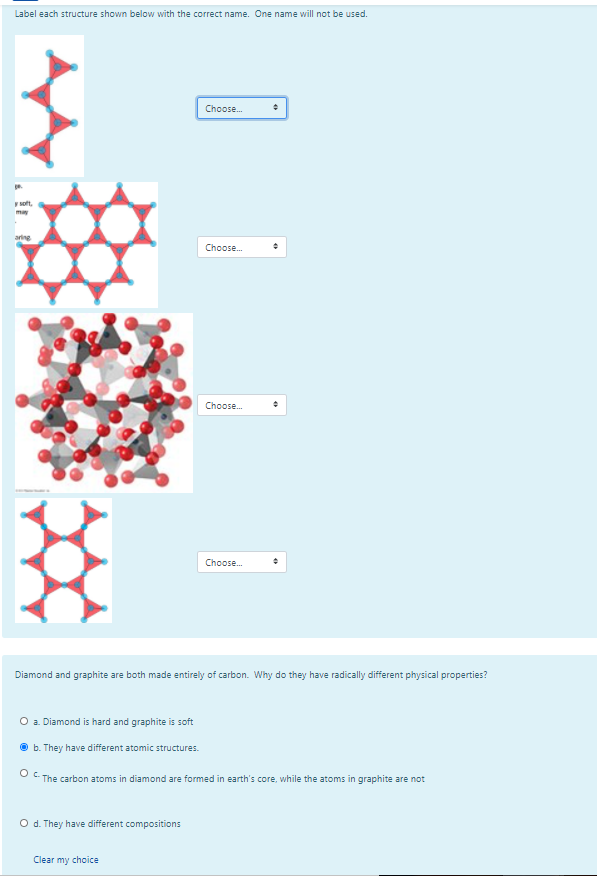
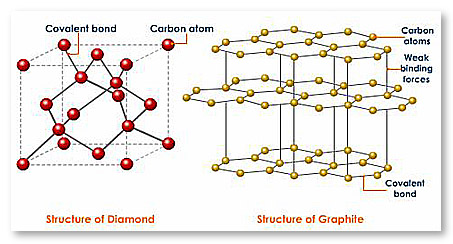
Solved Label each structure shown below with the correct
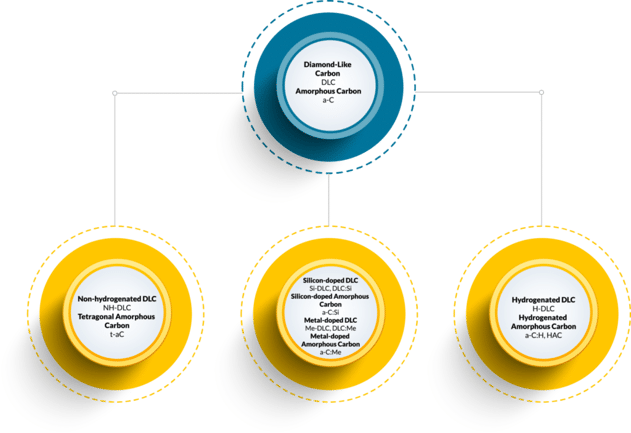
What is DLC, Also Known as Diamond Like Carbon Coating?

The Atomic Difference Between Diamonds and Graphite – Sustainable Nano
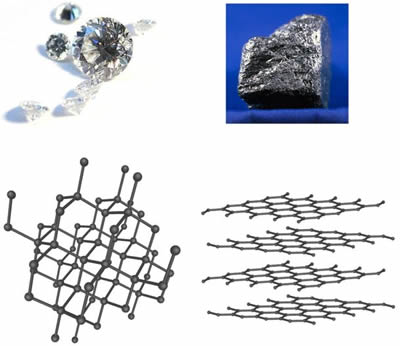
Diamond and Graphite

Why diamond and graphite have different physical properties but same chemical properties? What is the property called? - Quora
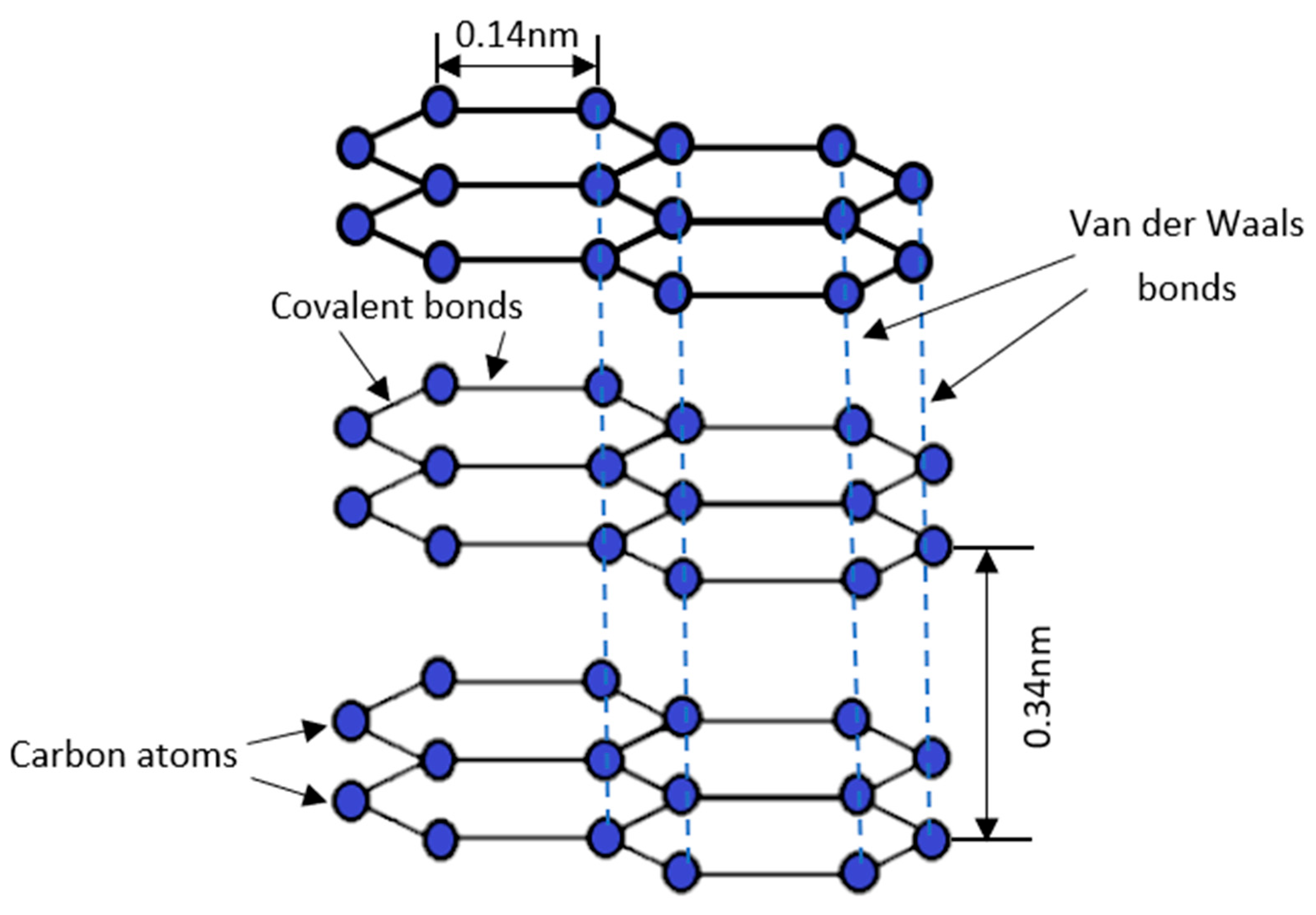
Materials, Free Full-Text
Hardness of Diamond - It's Hardest and NOT Toughest substance

Look the diagram and answer the following questions:i) What of structure do diamond and graphite have?ii) Why are diamonds used in cutting tools?iii) Why is graphite used in electrical circuits?iv) Name the

Differences Between Graphite and Diamond - javatpoint

Diamond Description

Narrow-gap, semiconducting, superhard amorphous carbon with high toughness, derived from C60 fullerene - ScienceDirect

Why is diamond hard and graphite brittle while both are made up of carbon atoms? A Difference in the melting point B Difference in the conductivity YOUR ANSWER C Difference in nature
Recommandé pour vous
 Graphite in pieces14 Jul 2023
Graphite in pieces14 Jul 2023 What is Carbon Graphite? - ROC Carbon14 Jul 2023
What is Carbon Graphite? - ROC Carbon14 Jul 2023 Graphite Pencil Tips and Techniques – Faber-Castell USA14 Jul 2023
Graphite Pencil Tips and Techniques – Faber-Castell USA14 Jul 2023 China Adjusts Export Restrictions for Graphite14 Jul 2023
China Adjusts Export Restrictions for Graphite14 Jul 2023 Top 10 Graphite-producing Countries14 Jul 2023
Top 10 Graphite-producing Countries14 Jul 2023 Specialty graphites for lithium-ion batteries14 Jul 2023
Specialty graphites for lithium-ion batteries14 Jul 2023 Choosing the Right Graphite Sketching & Drawing Pencil14 Jul 2023
Choosing the Right Graphite Sketching & Drawing Pencil14 Jul 2023 What is the link between diamond, graphite and buckyballs14 Jul 2023
What is the link between diamond, graphite and buckyballs14 Jul 2023- What Are the Uses of Graphite?14 Jul 2023
 Scientists solve puzzle of turning graphite into diamond14 Jul 2023
Scientists solve puzzle of turning graphite into diamond14 Jul 2023
Tu pourrais aussi aimer
 Ciseaux Fiskars universels pour gaucher 21 cm - Self Tissus14 Jul 2023
Ciseaux Fiskars universels pour gaucher 21 cm - Self Tissus14 Jul 2023 Tableau en Liège cadre bois, fixation fournie, livré avec 6 épingles - Format : L90 x H60 cm14 Jul 2023
Tableau en Liège cadre bois, fixation fournie, livré avec 6 épingles - Format : L90 x H60 cm14 Jul 2023 Horloge-contacteur-chauffe-eau14 Jul 2023
Horloge-contacteur-chauffe-eau14 Jul 2023 Nvidia Shield TV Pro full review 2023 -BEST Android TV Box 202314 Jul 2023
Nvidia Shield TV Pro full review 2023 -BEST Android TV Box 202314 Jul 2023 Carafe Filtrante Brita à Prix Carrefour14 Jul 2023
Carafe Filtrante Brita à Prix Carrefour14 Jul 2023 LEGO 10941 Mickey & Minnie Birthday Train - LEGO DUPLO14 Jul 2023
LEGO 10941 Mickey & Minnie Birthday Train - LEGO DUPLO14 Jul 2023 Glow up planner - France14 Jul 2023
Glow up planner - France14 Jul 2023- BOSS - Bracelet en cuir tressé marron avec fermoir à anneau en D14 Jul 2023
 Accessoires BDSM & Fetish - Sex Shop Suisse14 Jul 2023
Accessoires BDSM & Fetish - Sex Shop Suisse14 Jul 2023 Décoration d'anniversaire pat'patrouille, assiettes, gobelets en papier, vaisselle, bannière, ballons pour chiens et chasses, fournitures de fête14 Jul 2023
Décoration d'anniversaire pat'patrouille, assiettes, gobelets en papier, vaisselle, bannière, ballons pour chiens et chasses, fournitures de fête14 Jul 2023

