c) 20,000 200000 13. An element, X, have three isotopes 22X. The percentage abundance of its average atomic mass of the element percentage abundance of 21X should be ((a) 9% (b) 8% (
Par un écrivain mystérieux
Last updated 17 juillet 2024

Click here:point_up_2:to get an answer to your question :writing_hand:c 20000let 20000013 an element x have three isotopes22x the percentage abundance ofits average atomic
Click here👆to get an answer to your question ✍️ -c- 20-000 200000 13- An element- X- have three isotopes 22X- The percentage abundance of its average atomic mass of the element percentage abundance of 21X should be -a- 9- -b- 8- -c- 10- -d- 0- have three isotopes 20X- 21X and age abundance of 20X is 90- and c mass of the element is 20-11-The
Click here👆to get an answer to your question ✍️ -c- 20-000 200000 13- An element- X- have three isotopes 22X- The percentage abundance of its average atomic mass of the element percentage abundance of 21X should be -a- 9- -b- 8- -c- 10- -d- 0- have three isotopes 20X- 21X and age abundance of 20X is 90- and c mass of the element is 20-11-The

An element exist in three isotopic form 40x, 41X and 42x

SOLVED: The element X has three naturally occurring isotopes. The

The average atomic mass of a sample of an element X is 16.2 u

Solved Consider an element Z that has two naturally

PDF) Precalculus Seventh Edition With the assistance of David C

An element X has three isotopes with atomic masses X-100 , X-101

U 5. Naturally occurring neon consists of three isotopes, 20Ne, 2

55. The atomic weight of clement X is 51.7. If element X consists

61. An element X has two isotopes 41X and 43X. If percentage
Element X has two major isotopes 69X and 71X which occur naturally

12345-Elements of Physical Metallurgy, PDF, Heat Treating

Consider an element Z that has two naturally occurring isotopes
Recommandé pour vous
- Solved A hypothetical element X has 3 naturally occurring14 Jul 2023
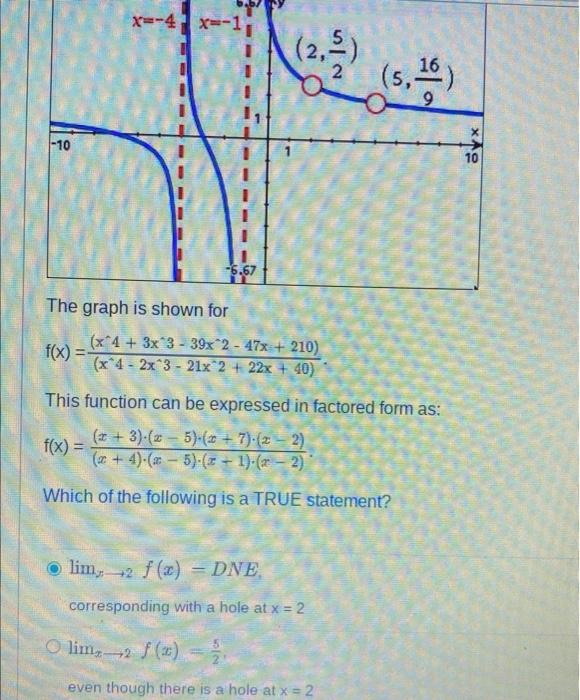
- Solved The graph is shown for14 Jul 2023
 Status of QNAP TS-21x/TS-22x support14 Jul 2023
Status of QNAP TS-21x/TS-22x support14 Jul 2023 Roster of Ohio University men and women who have served in the nation's wars - Ohio University Archives-General - Ohio University Libraries - Digital Archival Collections14 Jul 2023
Roster of Ohio University men and women who have served in the nation's wars - Ohio University Archives-General - Ohio University Libraries - Digital Archival Collections14 Jul 2023 2PCS Keyence Optical Fiber Sensor FU-21X FU-22X original14 Jul 2023
2PCS Keyence Optical Fiber Sensor FU-21X FU-22X original14 Jul 2023 原装基恩士FU-35FA 35FZ 45X 49X 65X 69X FU-21X 22X 23X 24X-Taobao14 Jul 2023
原装基恩士FU-35FA 35FZ 45X 49X 65X 69X FU-21X 22X 23X 24X-Taobao14 Jul 2023 Use the factor theorem to determine which of the following is NOT one of the factors of 4x^4 - 21x^3 -14 Jul 2023
Use the factor theorem to determine which of the following is NOT one of the factors of 4x^4 - 21x^3 -14 Jul 2023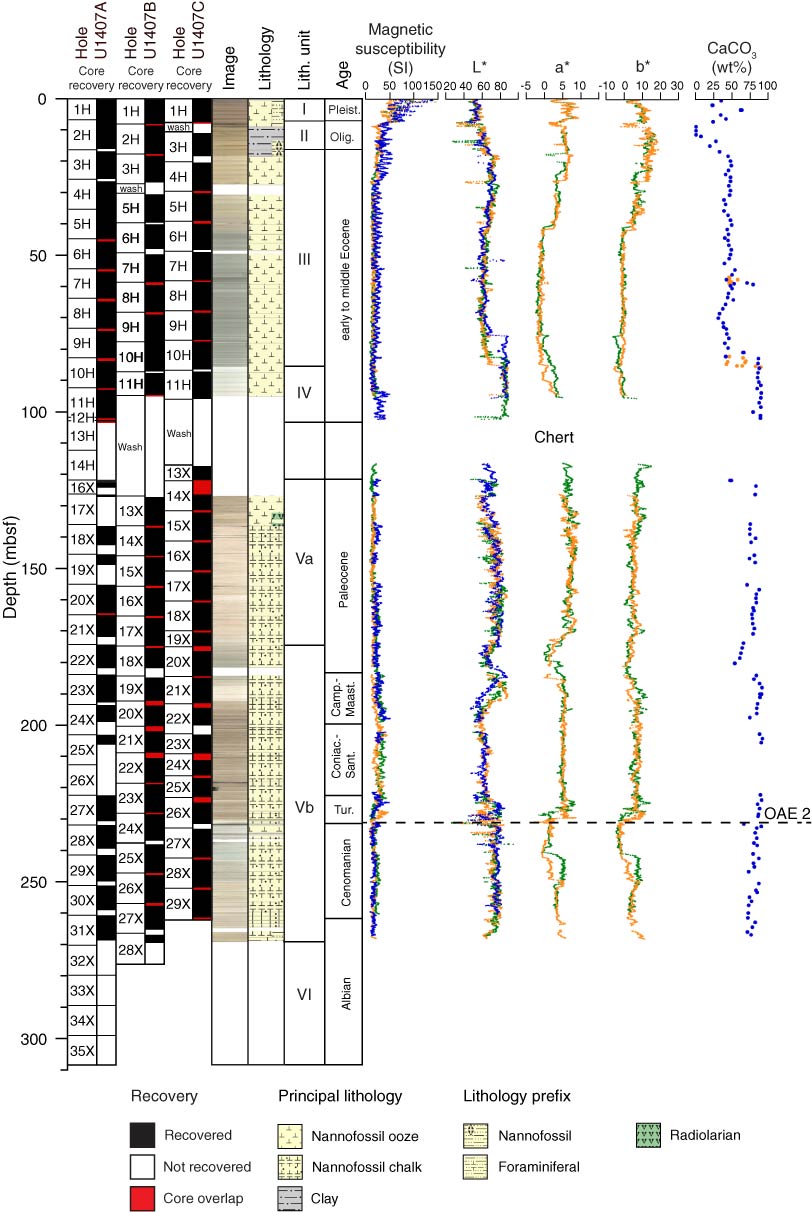 Proc. IODP, 342, Site U140714 Jul 2023
Proc. IODP, 342, Site U140714 Jul 2023 Factorise : 1. p2 + 5p + 6 2. x2 + 2x - 15 + 3m - 4 3. m 4. p2 - 10p + 24 1 + 11 11 + + + 5. 7-2-2 6. 7-22-8 7. x - 21x + 110 8. x2 - 21x + 90 9. X. - 22x + 117 10. X - 9x + 2014 Jul 2023
Factorise : 1. p2 + 5p + 6 2. x2 + 2x - 15 + 3m - 4 3. m 4. p2 - 10p + 24 1 + 11 11 + + + 5. 7-2-2 6. 7-22-8 7. x - 21x + 110 8. x2 - 21x + 90 9. X. - 22x + 117 10. X - 9x + 2014 Jul 2023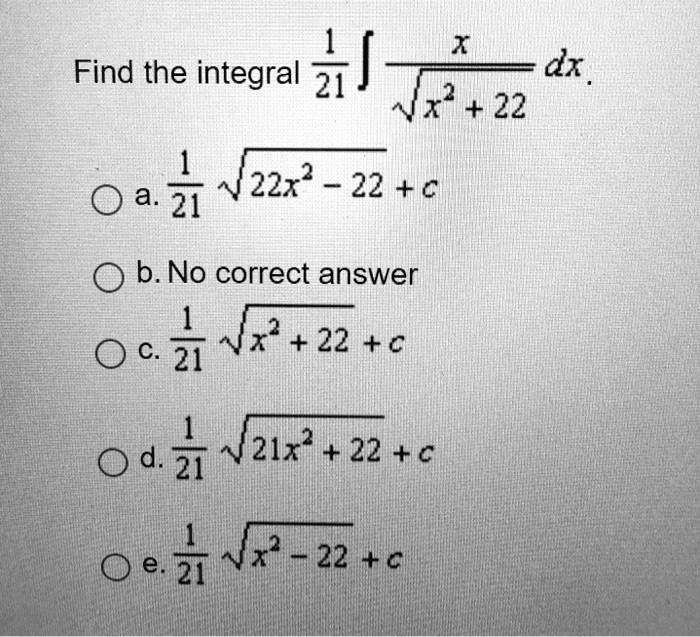 SOLVED: x Find the integral 21 ) dx Nx? + 22 1 Oa 21 2 22x 22 + C b. No correct answer 1 Nr + 22 + C O c 21 O d. 2 21x? + 22 + € 0 e: 3 4 22 + c14 Jul 2023
SOLVED: x Find the integral 21 ) dx Nx? + 22 1 Oa 21 2 22x 22 + C b. No correct answer 1 Nr + 22 + C O c 21 O d. 2 21x? + 22 + € 0 e: 3 4 22 + c14 Jul 2023
Tu pourrais aussi aimer
 Zitto Bumper Smart Cover Universel, Porte-téléphone portable en silicone avec cordon bandoulière réglable (Orange)14 Jul 2023
Zitto Bumper Smart Cover Universel, Porte-téléphone portable en silicone avec cordon bandoulière réglable (Orange)14 Jul 2023 LAMELLEUSE 701 W PJ7000J MAKITA14 Jul 2023
LAMELLEUSE 701 W PJ7000J MAKITA14 Jul 2023 Housse d'assise pour chaise haute bébé enfant gamme Ptit14 Jul 2023
Housse d'assise pour chaise haute bébé enfant gamme Ptit14 Jul 2023 Écouteurs serre-tête avec réduction de bruit adaptative réelle JBL14 Jul 2023
Écouteurs serre-tête avec réduction de bruit adaptative réelle JBL14 Jul 2023 PDF) Comparison of the safety and immunogenicity of an investigational and a licensed quadrivalent meningococcal conjugate vaccine in children 2-10 years of age14 Jul 2023
PDF) Comparison of the safety and immunogenicity of an investigational and a licensed quadrivalent meningococcal conjugate vaccine in children 2-10 years of age14 Jul 2023![Bouillotte Eau Chaude,9 Pcs Petite Bouillotte Bouteille D'Eau Chaude Bouillotte Enfant Pvc Bouillottes Bouillotte Douce Bouil[k1708] - Cdiscount Santé - Mieux vivre](https://www.cdiscount.com/pdt2/3/6/0/1/700x700/auc1701899569360/rw/bouillotte-eau-chaude-9-pcs-petite-bouillotte-bout.jpg) Bouillotte Eau Chaude,9 Pcs Petite Bouillotte Bouteille D'Eau Chaude Bouillotte Enfant Pvc Bouillottes Bouillotte Douce Bouil[k1708] - Cdiscount Santé - Mieux vivre14 Jul 2023
Bouillotte Eau Chaude,9 Pcs Petite Bouillotte Bouteille D'Eau Chaude Bouillotte Enfant Pvc Bouillottes Bouillotte Douce Bouil[k1708] - Cdiscount Santé - Mieux vivre14 Jul 2023 How to Change PC RGB Colors: 10 Easy Ways14 Jul 2023
How to Change PC RGB Colors: 10 Easy Ways14 Jul 2023 Jeu de quilles en bois bowling pour enfants 12 pièces - Maison Futée14 Jul 2023
Jeu de quilles en bois bowling pour enfants 12 pièces - Maison Futée14 Jul 2023 Baume universel certifié bio I Mustela14 Jul 2023
Baume universel certifié bio I Mustela14 Jul 2023 Panier Garnis vin blanc SANS ALCOOL - Est cadeaux14 Jul 2023
Panier Garnis vin blanc SANS ALCOOL - Est cadeaux14 Jul 2023